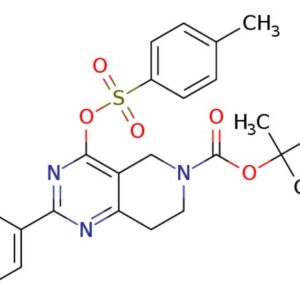
Description
Formulated with purified, intact virus particles that have been chemically modified to render them non-infectious and refrigerator stable
- Each vial contains 1.0mL of VZV NATtrol at various concentrations
- Supplied in a purified protein matrix that mimics the composition of a true clinical specimen
- Can be used to evaluate the performance of nucleic acid tests for determination of the presence of VZV DNA
Validation of clinical assays, development of diagnostic tests and training of laboratory personnel
| Certifications/Compliance | ISO, 13485 |
| Concentration | 50000 copies/mL |
| Content And Storage | 2°C to 8°C |
| Form | Solution |
| Media Type | Vial |
| Product Type | NATtrol Virus Strain Control |
| Formulation | Formulated with purified, intact virus particles |
| For Use With (Application) | Determination of presence of VZV RNA, validation of clinical assays, development of diagnostic tests and training of laboratory personnel |
| Quantity | 1 mL |